ISO 13485 MEDICAL DEVICE MANAGEMENT SYSTEM
Become ISO 13485 Certified Today!
ISO 13485:2016 Medical Device Management Systems certification is essential for medical device manufacturers and suppliers, providing a framework to ensure the consistent, safe, and compliant design, production, and delivery of medical devices while supporting risk management and best manufacturing practices.
ISO 13485 Certification & BEYOND

Our Services at PA Certs
Certification - We provide certification to ISO 13485:2016.
Stage 1 Audits - We provide stage 1 audits and assessments to prepare you for the certification process.
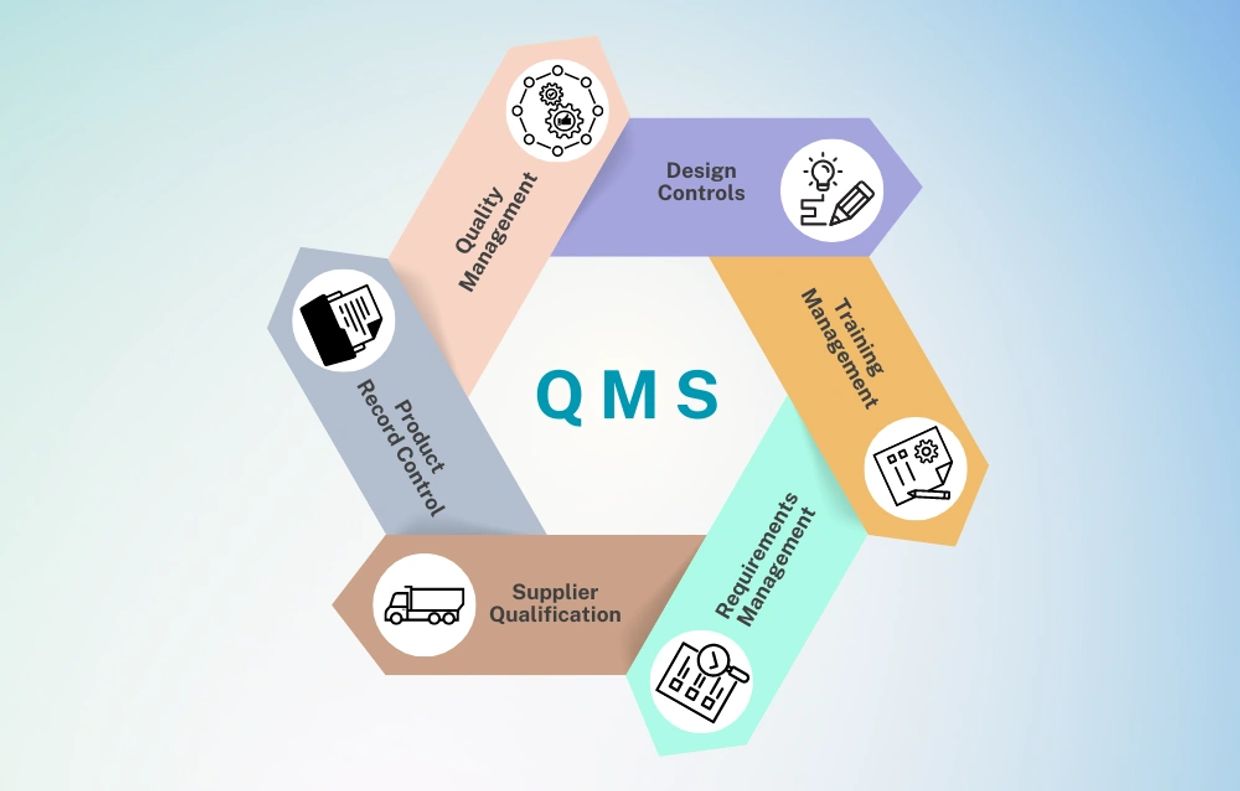
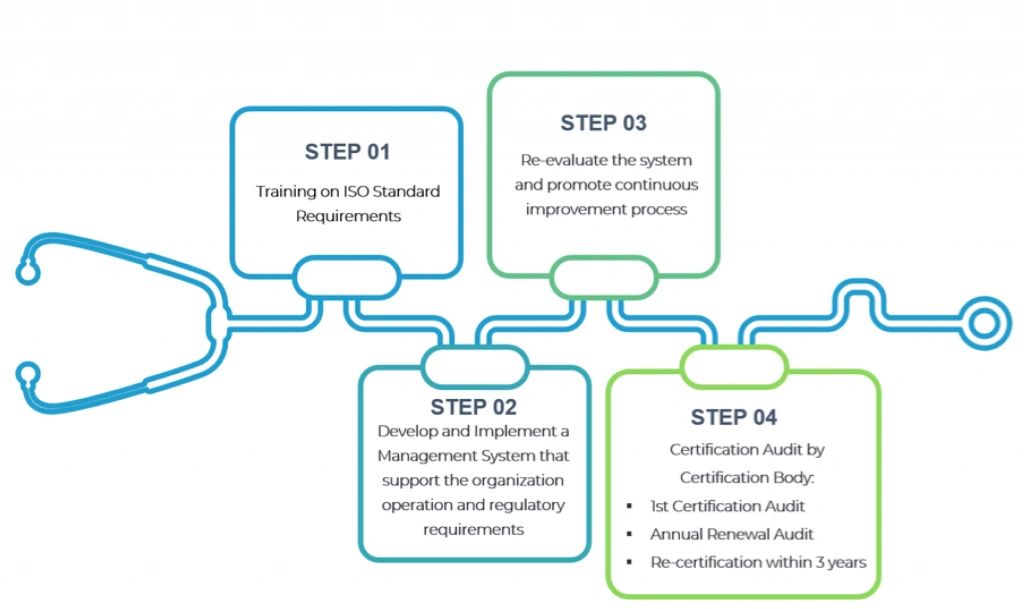
OUR PATH TO SUCCESS
Our ISO 13485 Medical Device Management Systems certification process is expertly designed to deliver maximum effectiveness, guiding you seamlessly to success.
Drawing on over 25 years of experience across diverse industries, we deliver a customized approach designed specifically for you.
Step 1 - Contract Agreement
We start by creating a certification agreement that defines the scope, timelines, and terms for your ISO 13485 Medical Device Management System registration. Once the agreement is signed, you'll be provided with information regarding system standards and guidelines.
Step 2 - Scheduling the Audit
Our team will be in contact with you to start scheduling the audit certification process. Information will be gathered regarding operations and procedures to help understand your business, this helps plan a staged audit approach customized to your needs.
Step 3 - Pre-Audit
Our services support medical device manufacturers in optimizing quality and regulatory infrastructures, strengthening compliance and data protection, and improving cost efficiency through expert, business-specific recommendations.
Step 4 - Audit
The detailed ISO 13485 certification audit takes place on-site. We assess the effectiveness of the information security management system by reviewing documentation, observing processes on-site, and conducting interviews with employees.
Step 5 - Technical Review and Issuing the Certification
Once the auditors have submitted their reports to our Registration group for secondary review, we issue your ISO 13485 certificate. Your business will be recognized in our register of certified organizations.
Step 6 - Ongoing Surveillance Audits
Throughout the 3-year certification period, we arrange annual surveillance audits to ensure your information security management system continues to comply with ISO 13485 medical device standards.
our HIGHLY SKILLED TEAM WILL HELP GUIDE YOU THROUGH THE ENTIRE CERTIFICATION PROCESS!
Contact Us
Streamlining COMPLIANCE WITH INTERNATIONALLY RECOGNIZED MANAGEMENT SYSTEMS.
Copyright © 2024 PACerts - All Rights Reserved.
Powered by GoDaddy
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.